2023-12-25
On December 13, 2023, the first domestically developed implantable cardiac defibrillator (ICD) by Singular Medical, the Anbo ICD, embarked on a prospective, multicenter confirmatory trial. This trial, led by Professor Xu Wei of the Cardiology Department at Drum Tower Hospital of Nanjing, Vice President of the Arrhythmia Technology Branch, China Association of Medical Equipment, head of the domestic ICD clinical expert group, and Vice President of the Arrhythmia Committee of the Chinese Medical Doctor Association, marked a milestone with the successful enrollment of the first participant on December 19, 2023. This breakthrough signifies a new starting point for China in the field of cardiac defibrillation technology.

In recent years, the incidence of cardiovascular diseases and the rate of sudden cardiac death in China have remained high. The ICD, as an advanced technology medical device integrating multidisciplinary technologies, is currently the only effective treatment method recommended by both international and domestic medical guidelines for the prevention of sudden cardiac death. The ICD, described as an "ambulance inside the body," can restore normal cardiac rhythm by pacing and electrical defibrillation in cases of abnormal cardiac electrical activity. While the implantation rate of ICDs in the United States is 280 per million people, China's rate is only 3.61 per million. In Asia, Japan's rate is 51.79 per million, with Thailand and Malaysia also exceeding China.
Despite the clear clinical demand, no domestic ICD had passed market approval until now. Suzhou Singular Medical Co., Ltd., through tireless efforts and commitment to local independent R&D, developed the Anbo ICD. It has successfully passed type testing, short-term and long-term animal experiments, single-center acute human clinical studies, and has been reviewed and entered into the special review process (the "green channel") by the National Medical Products Administration. This is the first ICD product to enter the innovative special review program.

Professor Zhang Shu, President of the Arrhythmia Technology Branch, China Association of Medical Equipment, President of the Arrhythmia Committee of the Chinese Medical Doctor Association, and Director of the National Cardiovascular Disease Center at Fuwai Hospital of the Chinese Academy of Medical Sciences summarized the development process of the domestic ICD at the launch meeting. He expressed high hopes for this large-scale clinical study and noted that the ICD, with its fully independent intellectual property rights, will enhance China's international status in this field.
The first participant enrolled in the study was a middle-aged male with dilated cardiomyopathy. Professor Xu Wei's team expertly guided the intraoperative defibrillation testing, ensuring its successful execution. All metrics were outstanding, achieving the study's objectives seamlessly on the first attempt. The participant has completed follow-up and was successfully discharged from the hospital.
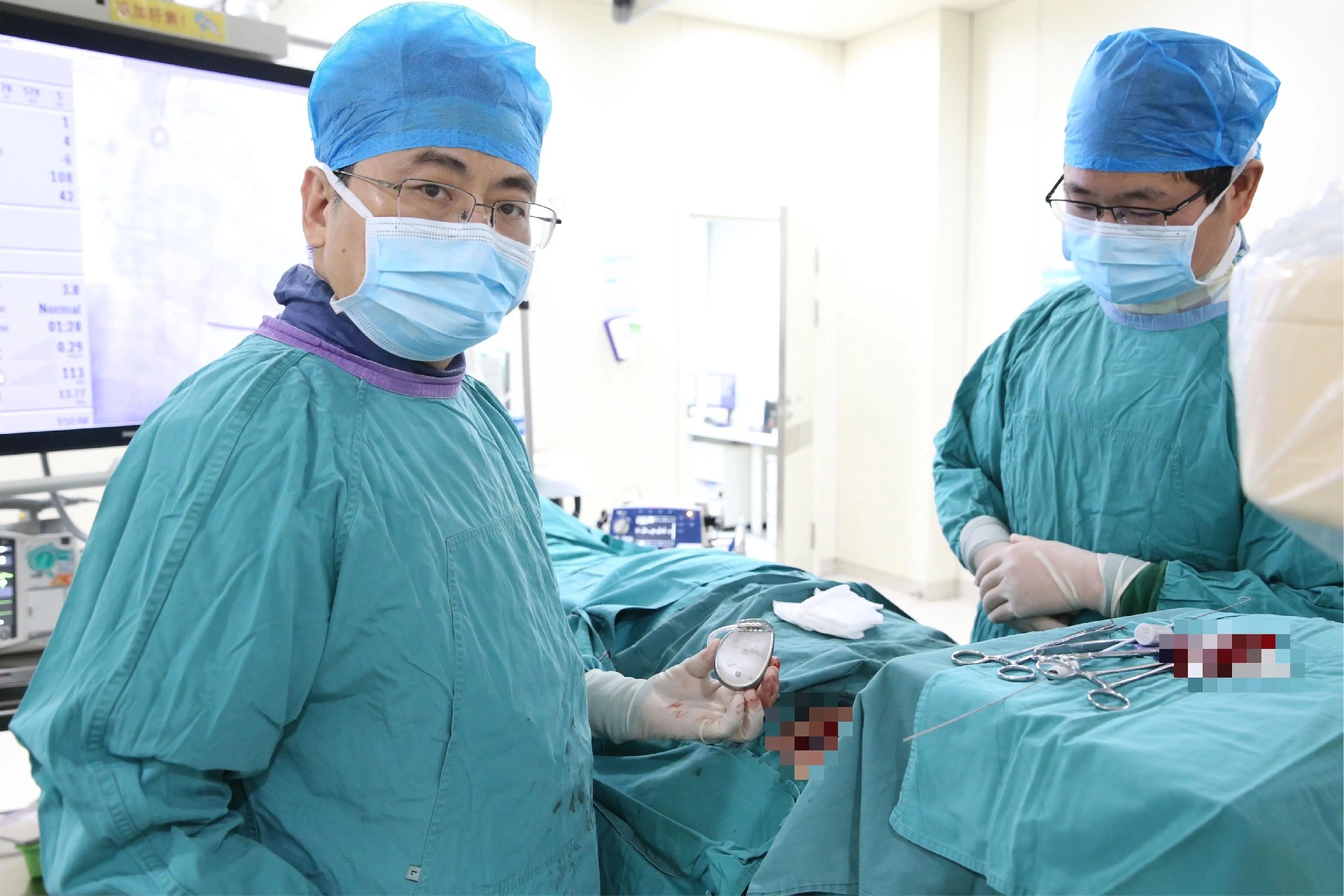
The confirmatory trial of Singular Medical's Anbo ICD aims to verify the safety and effectiveness of the first domestically produced ICD, collaborating with multiple centers nationwide to lay a solid foundation for clinical application. Despite the challenge, determination is the key to ensuring success. The company will continue to strive to build a strong engine for domestically produced high-end medical devices for arrhythmia, bringing Chinese solutions to more patients.
Company Introduction
Suzhou Singular Medical Co., Ltd. is a high-tech company focused on the development and industrialization of medical devices for Cardiac Rhythm Management (CRM).The company's headquarters are located in the Medical Device Industrial Park within the High-Tech Zone of Suzhou, with a 2,800 square meter manufacturing facility. Singular Medical also has research and development centers in Beijing, Irvine (USA), and Minnesota (USA). Starting with the most technically challenging product in the CRM field, the Implantable Cardiac Defibrillator (ICD), Singular Medical has built a comprehensive CRM technology platform. The company is growing to become a supplier of a full range of CRM products in China, capable of providing a complete solution for patients with cardiac arrhythmias, ranging from monitoring and diagnosis to treatment and postoperative follow-up.